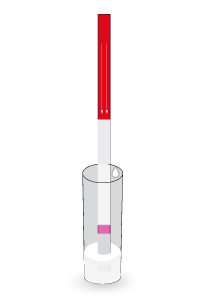
HOW DOES IT HELP YOU?
QuinoScan helps you to identify upon arrival if the milk tank is contaminated with quinolones at the plant in a simple way and in just 6 minutes. Once the operator runs the test at the reception, or even on the truck, the results will be automatically received in-real time allowing to make quick decisions.
QuinoScan analysis at the farm help you avoid industry penalties. Just by adding the milk you can control the tank in a few minutes before it is collected. In addition, the automatic reading with IRIS reader will allow you to share the results with the processing plant to daily guarantee the quality of your milk.