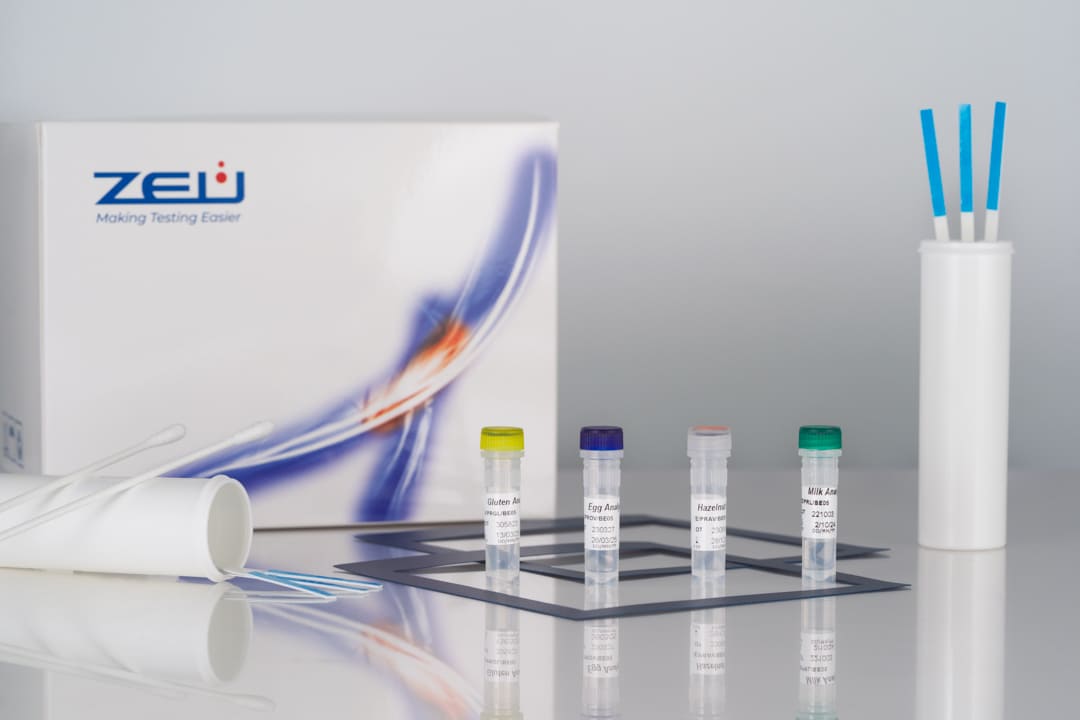
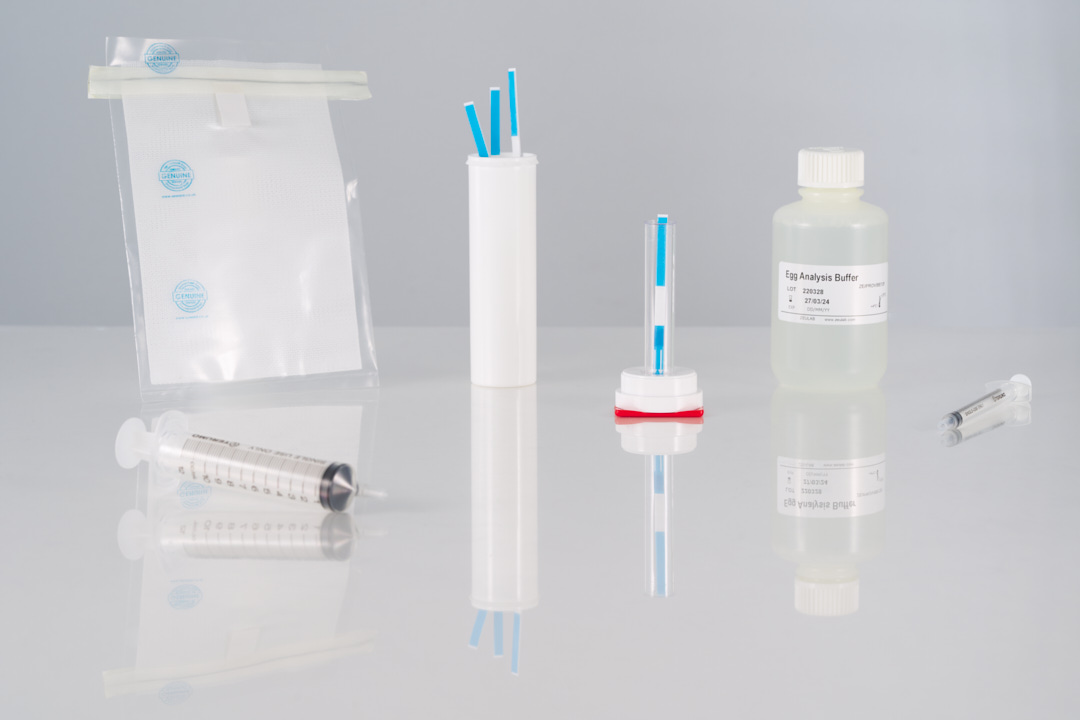
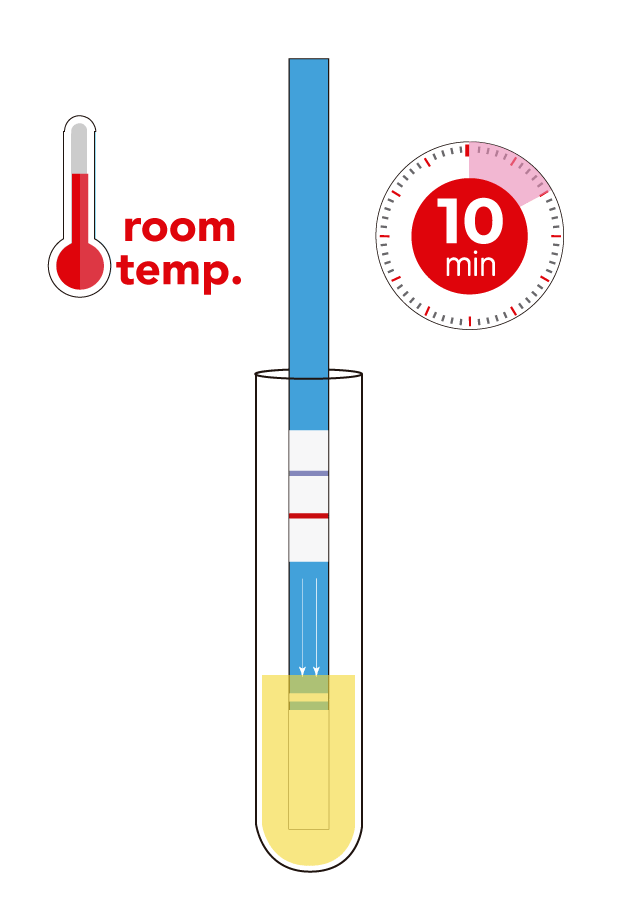
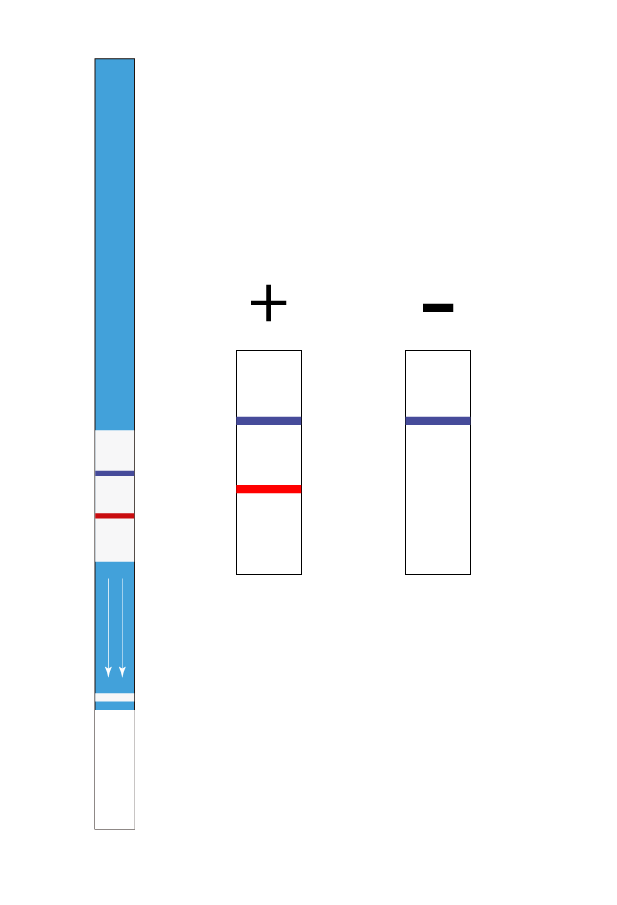- Limit of detection: 1.2 ppm of soy proteins.
- Limit of detection in surfaces: 0.7 µg of soy proteins.
- Assay time: 10 min.
Validations:
Internal validation following International guidelines:
Appendix M: Validation Procedures for Quantitative. Food Allergen ELISA Methods: Community Guidance and Best Practices. AOAC 2012 (http://www.eoma.aoac.org/app_m.pdf).
Appendix F: Guidelines for Standard Method Performance Requirements. Official Methods of Analysis (2016), AOAC INTERNATIONAL, Rockville, MD, USA (http://www.eoma.aoac.org/app_f.pdf)
Standard Method Performance Requirements (SMPRs®) for Detection and Quantitation of Selected Food Allergens. AOAC SMPR 2016.002. AOAC INTERNATIONAL, Rockville, MD, USA (https://www.aoac.org/aoac_prod_imis/AOAC_Docs/SMPRs/SMPR%202016_002.pdf).
Supporting bibliography for validation of antibodies and extraction methods of food samples:
Segura-Gil et al. (2020). Influence of different extraction conditions on the detection of glycinin and β-conglycinin in model processed foods by ELISA. Food Additives and Contaminats Part A.
Segura-Gil et al. (2019). Development of sandwich and competitive ELISA formats to determine β-conglycinin: Evaluation of their performance to detect soy in processed food. Food Control (103) 78-85.
Legislation:
Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 Text with EEA relevance.