HOW DOES IT HELP YOU?
The IC Caprino Quanti test helps you to easily quantify the presence of goat’s milk in sheep’s milk upon arrival at the plant or before the production of your products. You can also carry out the check during milk collection, on the farm or in the truck.
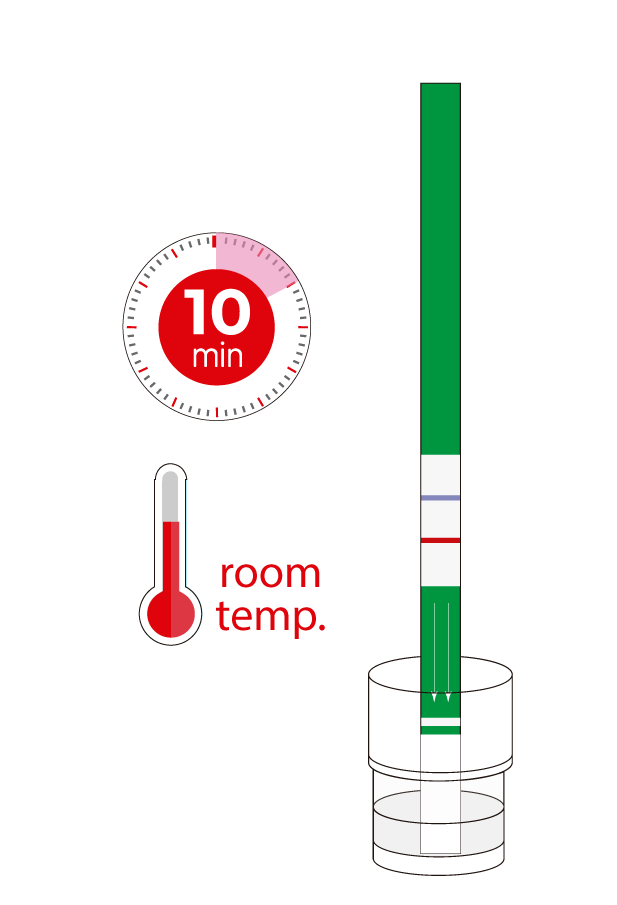
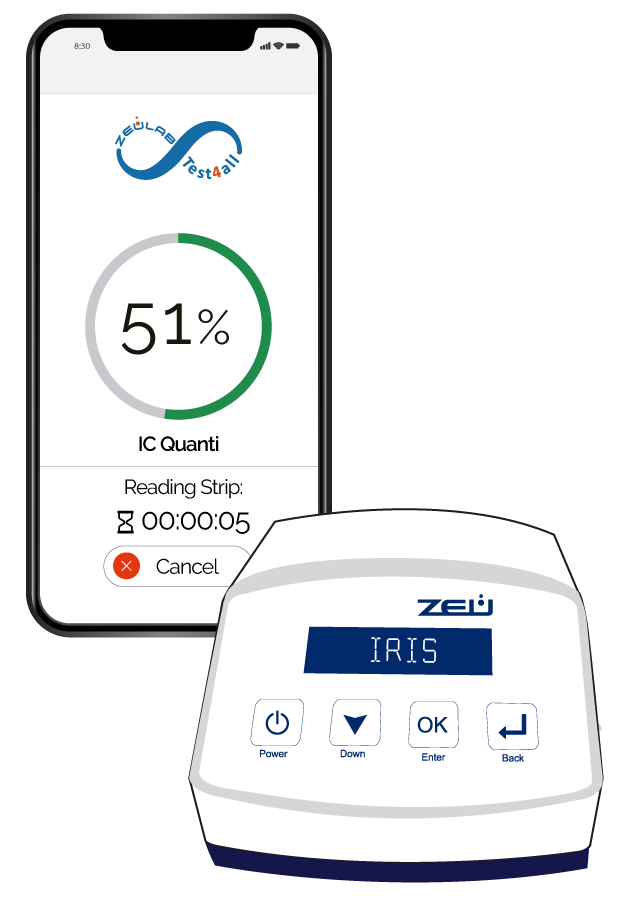
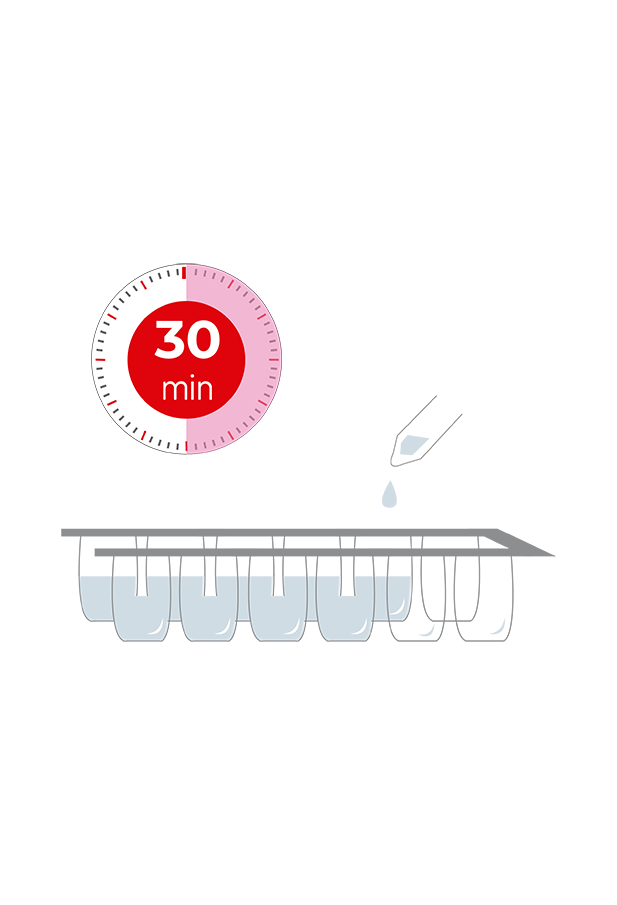
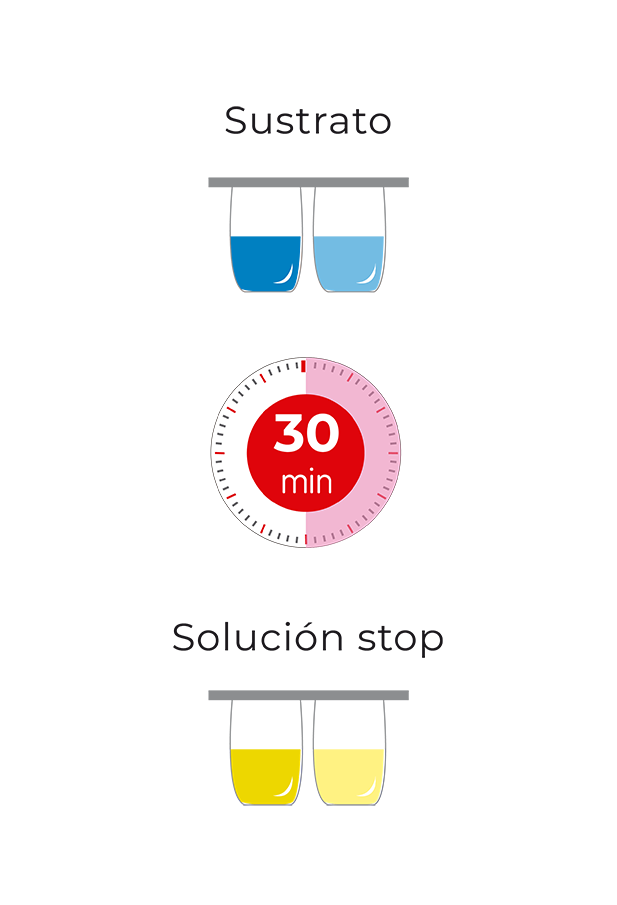
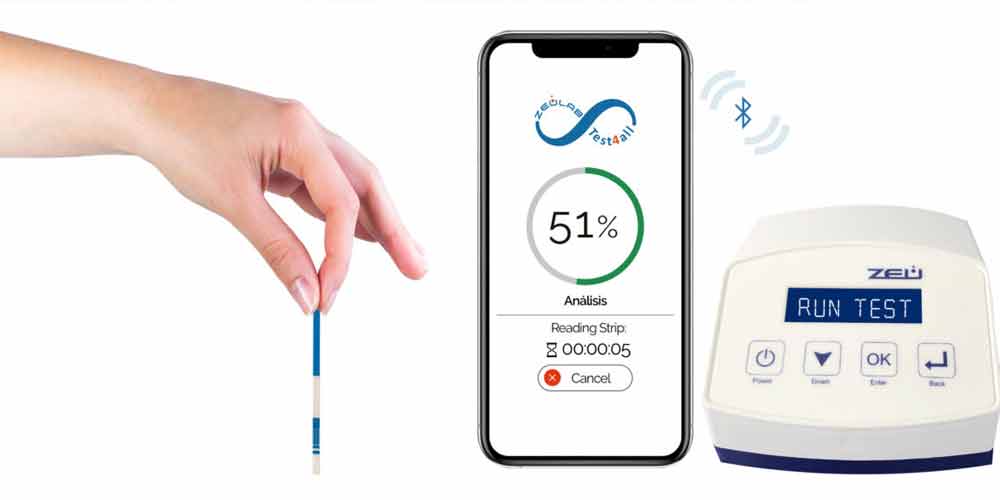
Check the correct cleanliness of tanks and installations to avoid cross-contamination. Just mix a drop of milk with a few drops of the analysis solution and dip the test strip. Thanks to the integration of the IC test on the Test4all platform you will be able to read the strip automatically, receive and store the results on your mobile or tablet in real time and ensure its full traceability.